Describe the Four Steps Used to Solve Stoichiometry Problems
B Use the mol ratio in the balanced reaction between the 2 compounds you are interested in. Express ideas share ideas challenge ideas accommodate ideas and apply ideas.

Solving Stoichiometry Problem Step By Step Mass To Mole Ppt Download
Hydrogen gas combines with oxygen gas to form water.

. What step must be performed before any stoichiometry problem is solved. Balance the equation 2. SOLVEDExplain why a balanced chemical equation is needed to solve a stoichiometric problem.
Calculate the moles using the ratios. Convert moles of wanted substance to desired units EXAMPLE. What step must be performed before any stoichiometry problem is solved explain.
Write the balanced equation for the reaction. There are four steps in solving a stoichiometry problem. C Find the grams of the compound you are looking for.
Find the actual masses. Mole- mole a Must ALWAYS begin with a balanced equation b Will have to use factor label method what you want goes on top what you wanna get rid of goes on bottom c one portion of the fence will be a molar ratio from your balanced equation. H2 g O2 g H2O.
For example consider the equation for the reaction between. Convert moles of wanted substance to desired units. Use the molar mass to do this.
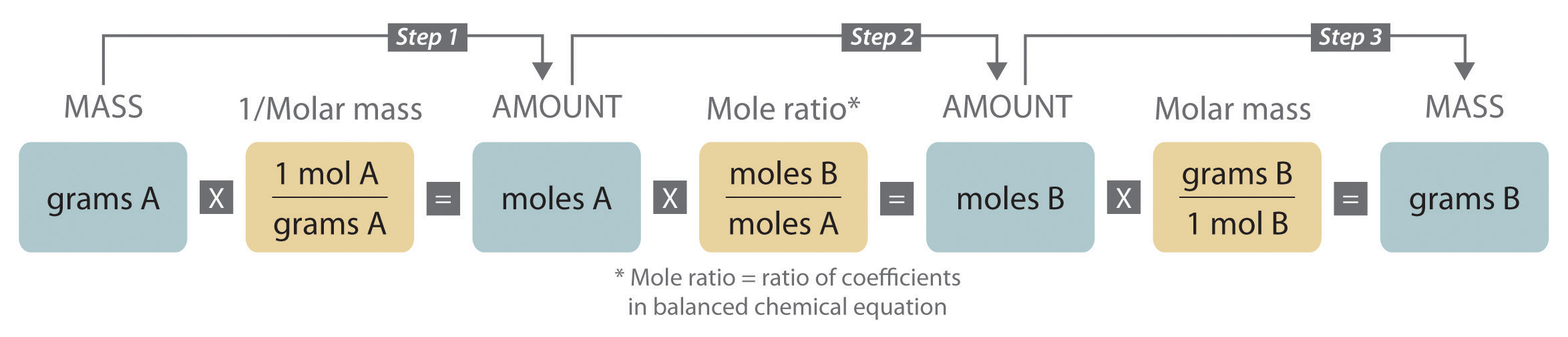
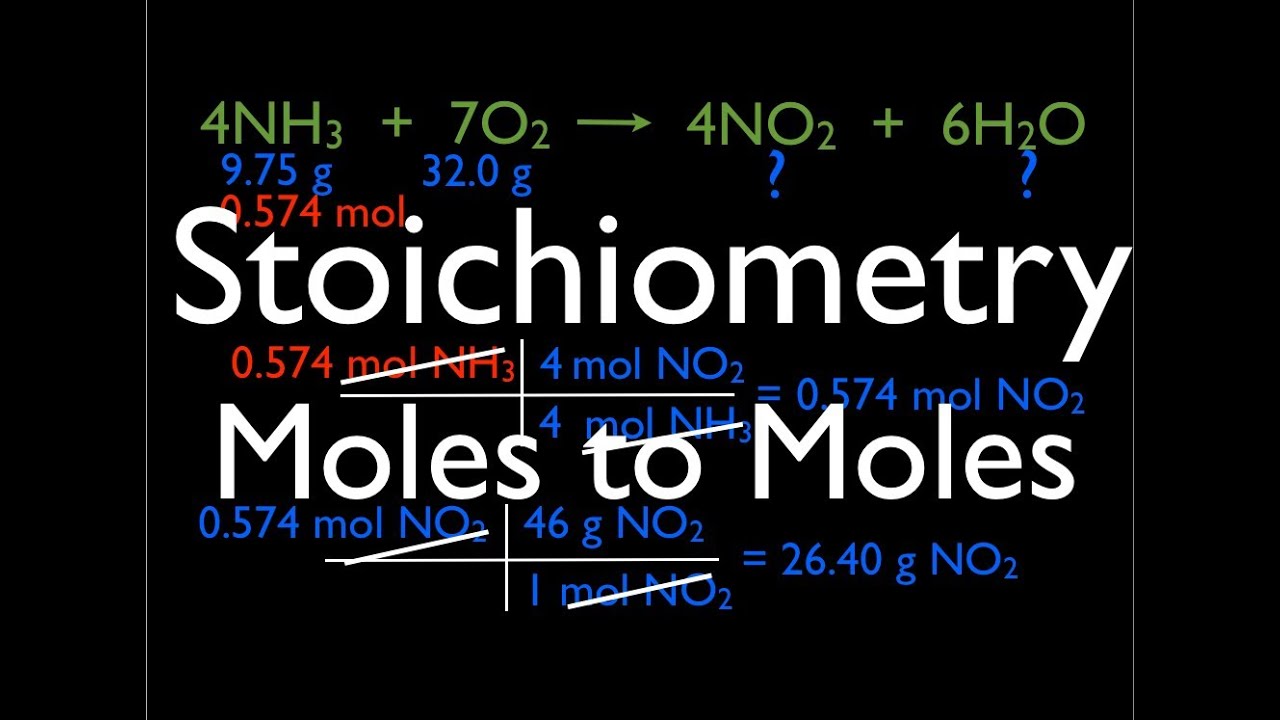
We can write a mole ratio for a pair of substances by looking at the coefficients in front of each species in the balanced chemical equation. Almost all stoichiometric problems can be solved in just four simple steps. H 2 2 O 2 32 H 2 O 18.
200g K are used in the reaction. Once you get this down its on to the actual stoichiometry. In this case we are given the mass of K 2 Cr 2 O 7 in 1 mL of solution which we can use to calculate the number of moles of K 2 Cr 2 O 7 contained in 1 mL.
Its rare for reactants to be present in. Convert grams of the substance given in the problem to moles. Do this by setting two molar ratios equal to each other with the unknown as the only value to solve.
The first step is to identify the ions present in each solution. Write down the relative atomic mass A r and the relative molecular mass M r for each substance in the equation. Calculate the moles of the given molg 200g K x 1 mol K 39g K 0513 mol K.
A common type of stoichiometric relationship is the mole ratio which relates the amounts in moles of any two substances in a chemical reaction. The only time you look at the balanced reaction is for step. Find moles of wanted substance using mole ratio 4.
051 mol K x 1mol H 2 2mol K 0266mol H 2. The sections below help explain key problem-solving steps. 2H 2 g O 2 g 2H 2 O l Step 2.
Up to 24 cash back FOUR STEPS TO SOLVE STOICHIOMETRIC PROBLEMS 1. Make sure you are working with a properly balanced chemical equation. What concentration what molarity.
Using the mole ratio calculate the moles of substance yielded by the reaction. How is a mole ratio used in stoichiometry. What volume how much solution.
Write the balanced chemical equation. Use molarity as a conversion factor to find the number of moles of any reactant or product in solution. Convert units of given substance to moles 3.
Solve for n n MV convert moles to grams if necessary. Convert any mass values in the problem into moles. Construct two ratios - one from the problem and one from the chemical equation and set them equal.
Seen in the coefficients. The equation must be balanced. The units involve five steps.
Using A r or M r change the moles in the equation to grams. 1 Write the balanced chemical reaction. Moles K2Cr2O7 1 mL 025mgK2Cr2O7 mL 1g 1000mg 1 mol 294.
A Find the mols of the compound with known mass. Convert the units of the given substance A to moles. H 1 O 16 M r.
C3H7NO2 O2 -- CO2 H2O NO2 1 475 3 35 1 4 C3H7NO2 O2 -- CO2 H2O NO2 4 19 12 14 4 Always remember to check your work. Balance The Equation Calculate the Ratios. Use the mole ratio to calculate the moles of wanted substance B.
Which of the following steps is unique to solving stoichiometry problems involving one or more substances in solution. To solve stoichiometry problems you must first do two very important things1 Write a balanced equation for the reaction2 Convert all amounts of products andor reactants in the question into. There are four steps involved in solving these problems.
Use molar proportion to determine unknown quantities of moles. List 4 steps used in solving stoichiometric problems. In the present work we describe an intervention consisting of the development of a series of stoichiometry learning units SLUs based on a conceptual change approach.
How many grams of water. Go back and review them if you need to because if you cant do that stuff you cant do stoichiometry. It is used as a conversion factor that relates the amts.
Convert the mole value you just found into mass using the molar mass of that substance. List the four steps used in solving stoichiometri. How is a mole ratio correctly expressed when its used to solve a stoichiometric problem.
2K2H 2 O 11 2K2KOH 11 2K1H 2 21 Step 2. The ratio from the problem will have an unknown x. Stoichiometry Test Chapter 11.
Diagnose the situation so that your focus is on the problem not just its symptoms. Helpful problem-solving techniques include using flowcharts to identify the expected steps of a process and cause-and-effect diagrams to define and analyze root causes. Up to 24 cash back 4 Types of Stoichiometry Problems.
If you struggled with those in class welcome to the club. According to Barnsford and Stein 1984 and Hayes 1981 the five basic approaches to problem solving are working backwards breaking a problem into parts working systematically solving problems by analogy and using procedural and conceptual knowledge. Convert units of a given substance to moles.
A In any stoichiometry problem the first step is always to calculate the number of moles of each reactant present. Solve for M. Find the Mass of the Given.
You will need to use molar ratios molar masses balancing and interpreting equations and conversions between grams and moles. Solve for V V n M. How are mole ratios used in chemical calculations.
2 Write a conversion equation. The Steps Involved in Solving Mass-Mass Stoichiometry Problems.

Step By Step Stoichiometry Practice Problems How To Pass Chemistry Youtube

Scaffolding The Factor Label Method In The Chemistry Classroom Science And Math With Mrs Lau Chemistry Classroom Science Classroom Chemistry Worksheets
3 6 Reaction Stoichiometry Chemistry Libretexts

Gas Stoichiometry Problems Youtube

Solving Stoichiometry Problems Ppt Video Online Download

6 5 Mole Mass And Mass Mass Problems The Basics Of General Organic And Biological Chemistry

Solving Stoichiometry Problem Step By Step Mass To Mole Ppt Download

What Is Stoichiometry How It Helps In Balancing Reactions The Engineering Projects

Free Steps To Solve Stoichiometry Problems Chemistry Reference Page Chemistry Solving High School Science

Chemistry Triangle Conversion Tool Moles Liters Molarity Atoms Chemistry Medicine Notes Science Chemistry

Stoichiometric Calculations Ck 12 Foundation
5 3 Stoichiometry Calculations Chemistry Libretexts

Solved List The Four Steps Used In Solving Stoichiometric Problems

What Is The Relationship Between A Mole And Avogadro S Number A Plus Topper Noofmolesformula Molar Mass Molecular Mass Mole
Reaction Stoichiometry Boundless Chemistry

How To Solve Reaction Stoichiometry Problems Mass Mass Mass Liter Etc Youtube
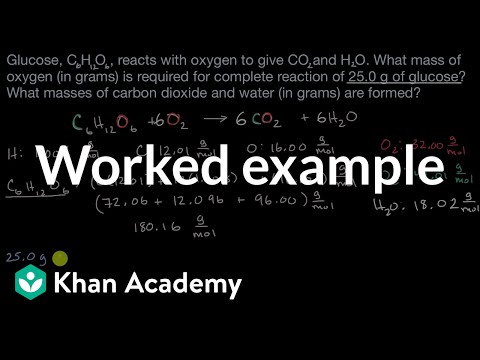
Calculating Amounts Of Reactants And Products Worked Example Video Khan Academy


Comments
Post a Comment